Leading Nutraceutical Company Specializing in High-Quality, Scientifically Backed Supplements
Natural, High-Quality Ingredients
Every supplement is rigorously researched and clinically tested for efficacy and safety. Our commitment to science ensures our products exceed industry standards, offering you trusted health solutions
Results-Driven
Our products are crafted to deliver real health improvements, with thousands of customers worldwide attesting to their effectiveness. Longevinex® is dedicated to enhancing your wellness journey with proven, transformative results.
Best Selling Products
Featured
“With over four decades of experience as an anti-aging physician, I wholeheartedly endorse the solutions Longevinex offers. Longevinex’s unwavering dedication to advancing health and wellness, expertly balanced with its emphasis on education and preventive care, is truly commendable. My collaborative partnership with Longevinex paves the way for individuals to embark on their journey towards a healthier, more vibrant future. I cordially invite you to explore our array of educational materials. You will be as impressed and empowered as I was on your unique health journey toward proactive optimal well-being.”
– Dr. William Clearfield

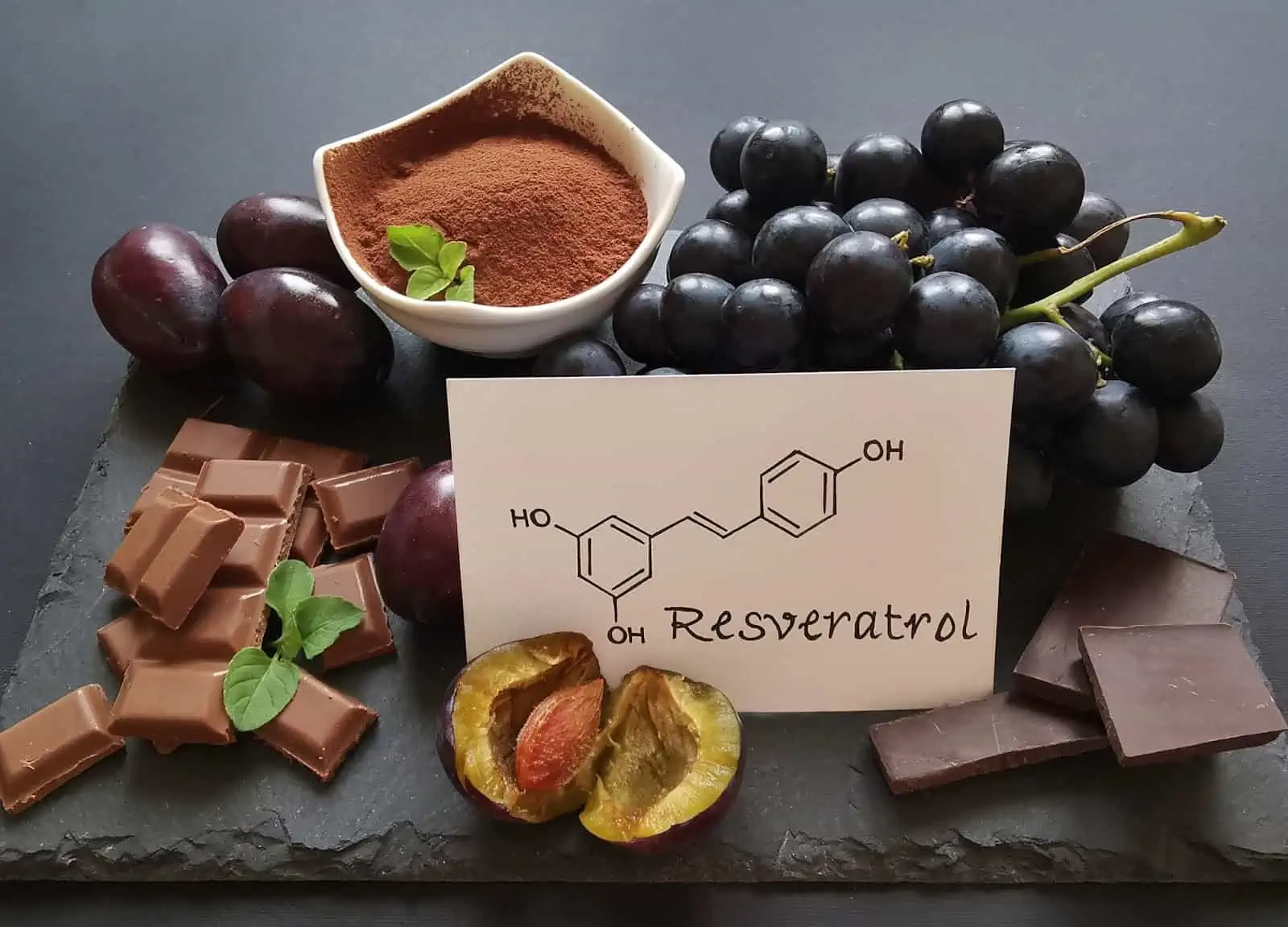
Setting the Gold Standard in Resveratrol Supplements Since 2004
Patented with DNA repair nucleotides
Synergistic array of molecules
Stabilized Resveratrol has a longer shelf life.
Stabilized with beta cyclodextrin
Opaque shell protects from light, heat, oxygen
Added nutrients
In addition to resveratrol’s potent anti-aging properties, we’ve enriched Longevinex® by adding additional nutrients to further enhance its synergistic anti-aging capabilities.
Backed By Science
Longevinex holds a US Patent for a nutraceutical matrix that enhances the absorption and stability of resveratrol, akin to a proprietary drug in clinical trials. This patented formula, celebrated and used by researchers, has undergone extensive testing and studies.
Scientifically proven
Leading Nutraceutical Company Specializing in High-Quality, Scientifically Backed Supplements
Founded in 2004, Longevinex®is a leading nutraceutical company specializing in high-quality, scientifically backed supplements. Our flagship resveratrol products, Longevinex® and Advantage® enhanced by a patented nutraceutical matrix, provide unparalleled absorption and bioavailability. Committed to promoting healthy aging, cardiovascular health, and overall well-being, Longevinex® continues to lead with innovation, research, and dedication to excellence.

I have found the health nutrient, Longevenix, provides a standardized, easily digested, form of resveratrol. In addition, the other ingredients in Longevinex , quercetin and fisetin also have potential health benefits. I do take other nutrients in my diet for health maintenance, but I put Longevinex at the top of that list. I recommend this dietary supplement to my family, friends and patients.
Choosing Between Longevinex® or Advantage®

Benefits
Promotes health and longevity
Ingredients
- Trans-resveratrol
- Quercetin
- Rice bran IP6
- Vitamin D3
- Nucleotides
- Beta cyclodextrin
- Fisetin
- Beta glucan
- Vitamin B1 as Benfotiamine
Encapsulation
Opaque vege caps to protect from light
Number of capsules per box
30
Serving size
1 capsule
Amount of Resveratrol
100 mg (produces 9-fold greater biological effect than equal dose of plain resveratrol due to synergistic action with other molecules
Price
- $34.95/box
- $104.85 Buy 3, get 1 Free($26.21/Box)

Benefits
Promotes health and longevity
+ youthful appearance
Ingredients
- Trans-resveratrol
- Quercetin
- Rice bran IP6
- Vitamin D3
- Nucleotides
Beta cyclodextrin + Fisetin
- Beta glucan
- Vitamin B1 as Benfotiamine
Lutein/zeaxanthin Hyaluronic acid
Encapsulation
Opaque vege caps to protect from light
Number of capsules per box
60
Serving size
2 capsules
Amount of Resveratrol
100 mg (produces 9-fold greater biological effect than equal dose of plain resveratrol due to synergistic action with other molecule
Price
$51.95/Box
$155.85 Buy 3, get 1 Free ($38.96/Box)
Explore Our Articles – Solutions and Wisdom from Medical Professionals
Introduction In a world where health is increasingly prioritized, the interplay of different body systems is more evident than ever. The link between heart health and brain function is crucial yet often overlooked. (1) For […]
Introduction The search for ways to live longer has led to the development of anti-aging supplements that aim to slow down the natural process of aging. Two such supplements that have gained attention are NMN […]
Introduction In today’s health-conscious society, resveratrol supplements are gaining increasing attention for their numerous health benefits. A naturally occurring compound mainly found in grape skins and red wine, resveratrol is praised for its antioxidant properties […]
Great Products
I have tried many competitor products but I always come back to Longevinex. I feel confident that this company has the best range of health and longevity products available in what is a very crowded market. Bottom line – they make quality reliable products that can and do provide numerous health benefits over time.
- – Thomas Cleary
Best supplement I have ever taken
I’ve been taking Longevinex daily since 2006. This has been a fountain of youth for me in addition to feeling energetic and healthy. I am a lab scientist and I can’t wait to read all the new finding on this product each time one is available to me, many thanks to Longevinx for a wonderful aide to my life!
- – Dani McDaniel
Highly Recommended
I’ve been taking this for many, many years. I recommend it to EVERYONE interested in staying healthy. My blood work is always great, I have energy and feel great each day. If I miss my daily supplement for any reason I feel sluggish.
HIGHLY RECOMMEND THIS PRODUCT!!
- Sandy C